Respiratory Syncytial Virus Diagnostics Market Size
Summary:
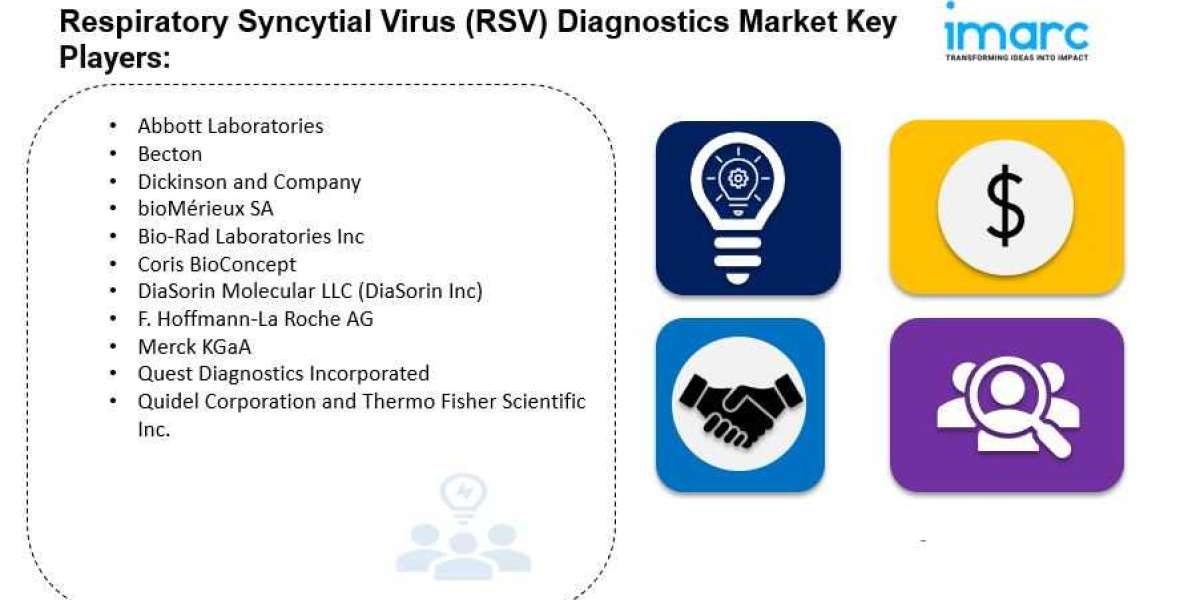
- The global respiratory syncytial virus diagnostics market size reached US$ 1,005.2 Million in 2023.
- The market is expected to reach US$ 2,072.3 Million by 2032, exhibiting a growth rate (CAGR) of 8.1% during 2024-2032.
- Based on the region, the market is segregated into North America (the United States and Canada), Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others), Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others), Latin America (Brazil, Mexico, and others), and Middle East and Africa.
- On the basis of Product, the market is segmented into direct fluorescent antibody (DFA) method, rapid antigen diagnostic test (RADTS), molecular diagnostics, chromatographic immunoassay, diagnostic imaging, gel microdroplets, flow cytometry, and others.
- Based on the end use, the market is classified into hospitals and clinics, laboratory, and others.
- The increasing emphasis on personalized medicine necessitates precise diagnostic tests to tailor treatments based on individual patient profiles and specific viral strains.
- Besides this, the growing trend of home healthcare and telemedicine for at-home diagnostic kits for respiratory syncytial virus (RSV) that provide convenience and facilitate remote patient monitoring, thus bolstering the market growth.
- Respiratory syncytial virus (RSV) is a viral infection of the lungs and respiratory tract that can lead to asthma, hospitalization, pneumonia, repeated infections, middle ear infections, and even death, driving the growth of the respiratory syncytial virus diagnostics market size.
Industry Trends and Drivers:
- The increasing prevalence of RSV infections:
The rising prevalence of RSV infections, particularly among vulnerable populations such as infants, the elderly, and individuals with compromised immune systems. RSV is a leading cause of lower respiratory tract infections in young children and can result in severe complications, including pneumonia and bronchiolitis. The annual incidence of RSV infections is substantial, with millions of hospitalizations occurring worldwide. As awareness of the impact of RSV on public health increases, healthcare providers are emphasizing the importance of accurate and timely diagnosis. This heightened focus drives demand for effective diagnostic tools that can quickly identify RSV infections, particularly during peak seasons when infections typically surge. The necessity for prompt diagnosis helps initiate appropriate clinical management and treatment, further strengthening the market growth.
- The growing awareness of respiratory diseases:
The increasing awareness of respiratory diseases among healthcare professionals and the general public is impelling the market growth. Public health initiatives and educational campaigns have significantly raised awareness of the risks associated with respiratory infections, including RSV. As healthcare systems prioritize respiratory health, there is a growing demand for comprehensive diagnostic solutions to improve patient outcomes. Healthcare providers are seeking reliable testing methods that can differentiate RSV from other respiratory pathogens, allowing for targeted treatment strategies. This trend has led to the development and implementation of guidelines recommending routine RSV testing in at-risk populations, further driving the demand for diagnostic solutions. Enhanced awareness is also reflected in increased funding for research and development (RD) aimed at improving diagnostics, thus aiding the market growth.
- Advancements in diagnostic technologies:
Innovative techniques such as molecular diagnostics, including polymerase chain reaction (PCR) tests and point-of-care (POC) testing, have significantly improved the accuracy and speed of RSV detection. These advancements enable healthcare providers to obtain results quickly, facilitating timely treatment decisions. Moreover, the development of user-friendly and portable diagnostic devices is making it easier for healthcare facilities, especially in remote or underserved areas, to implement effective RSV testing. These technological improvements are also lowering the costs associated with diagnostic testing, making them more accessible to a broader range of healthcare settings. As manufacturers continue to invest in RD to enhance the efficiency and accuracy of RSV diagnostics, it is supporting the market expansion.
Request for a sample copy of this report: https://www.imarcgroup.com/respiratory-syncytial-virus-diagnostics-market/requestsample
Respiratory Syncytial Virus Diagnostics Market Report Segmentation:
Breakup By Product:
- Direct Fluorescent Antibody (DFA) Method
- Rapid Antigen Diagnostic Test (RADTs)
- Molecular Diagnostics
- Chromatographic Immunoassay
- Diagnostic Imaging
- Gel Microdroplets
- Flow Cytometry
Breakup By End Use:
- Hospitals and Clinics
- Laboratory
- Others
Based on the end use, the market is classified into hospitals and clinics, laboratory, and others.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
On the basis of the region, the market is segregated into North America (the United States and Canada), Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others), Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others), Latin America (Brazil, Mexico, and others), and Middle East and Africa.
Top Respiratory Syncytial Virus Diagnostics Market Leaders:
The respiratory syncytial virus diagnostics market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
- Abbott Laboratories
- Becton
- Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Coris BioConcept
- DiaSorin Molecular LLC (DiaSorin Inc)
- Hoffmann-La Roche AG
- Merck KGaA
- Quest Diagnostics Incorporated
- Quidel Corporation and Thermo Fisher Scientific Inc.
Browse full report with TOC List of Figures: https://www.imarcgroup.com/request?type=reportid=4048flag=C
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145